Annex 1: CLINICAL TRIAL APPLICATION FORM (CTA) To be completed by Applicants for all Clinical Trials Study Title: Protocol No:
Guidelines on applications for authorisation to conduct toxicological and pharmacological trials for the purpose of assessing th

Solve Expiry Labels, DtP, and Timelines for EU 536/2014 Clinical Trials Regulation | Healthcare Packaging

Guidance Notes for Applicants of the Certificate for Clinical Trial on Medical Device - PDF Free Download
Page 1 of 5 MONITORING SERVICES FOR A CLINICAL TRIAL EXP_17_2019 Background The Barcelona Institute for Global Health, ISGlobal
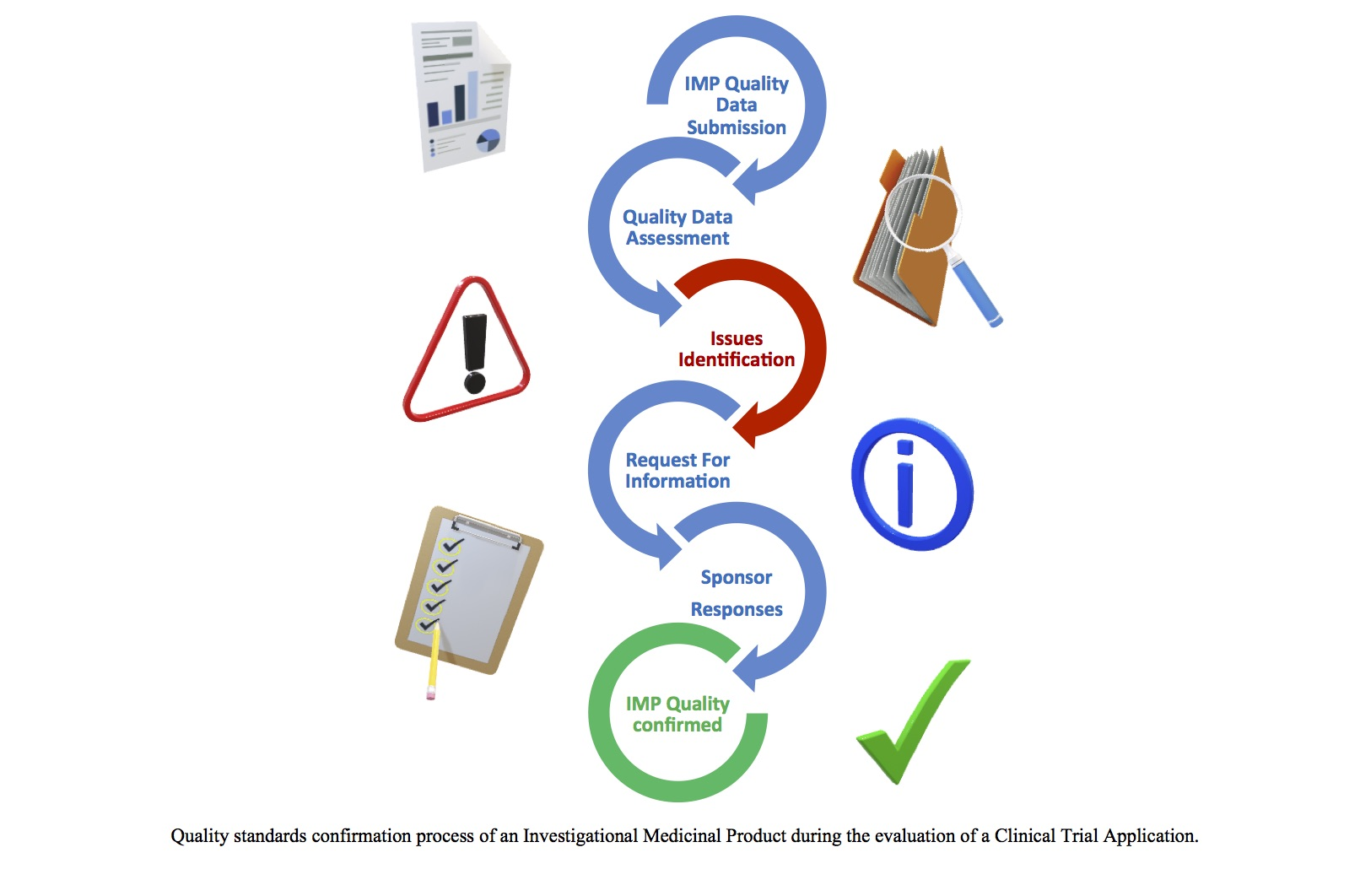
Annex 1: Clinical trial Application Form The questions in this form for the request for authorisation from the Competent Authori

Guidance Document: Part C, Division 5 of the Food and Drug Regulations “Drugs for Clinical Trials Involving Human Subjects” (GUI-0100) - Canada.ca
Request for Proposal Clinical Trial Supplies Services to support a phase 2 clinical trial in Eumycetoma

Clinical trials were missing from regulatory documents of extended-release methylphenidate for ADHD in adults: a case study of public documents - Journal of Clinical Epidemiology
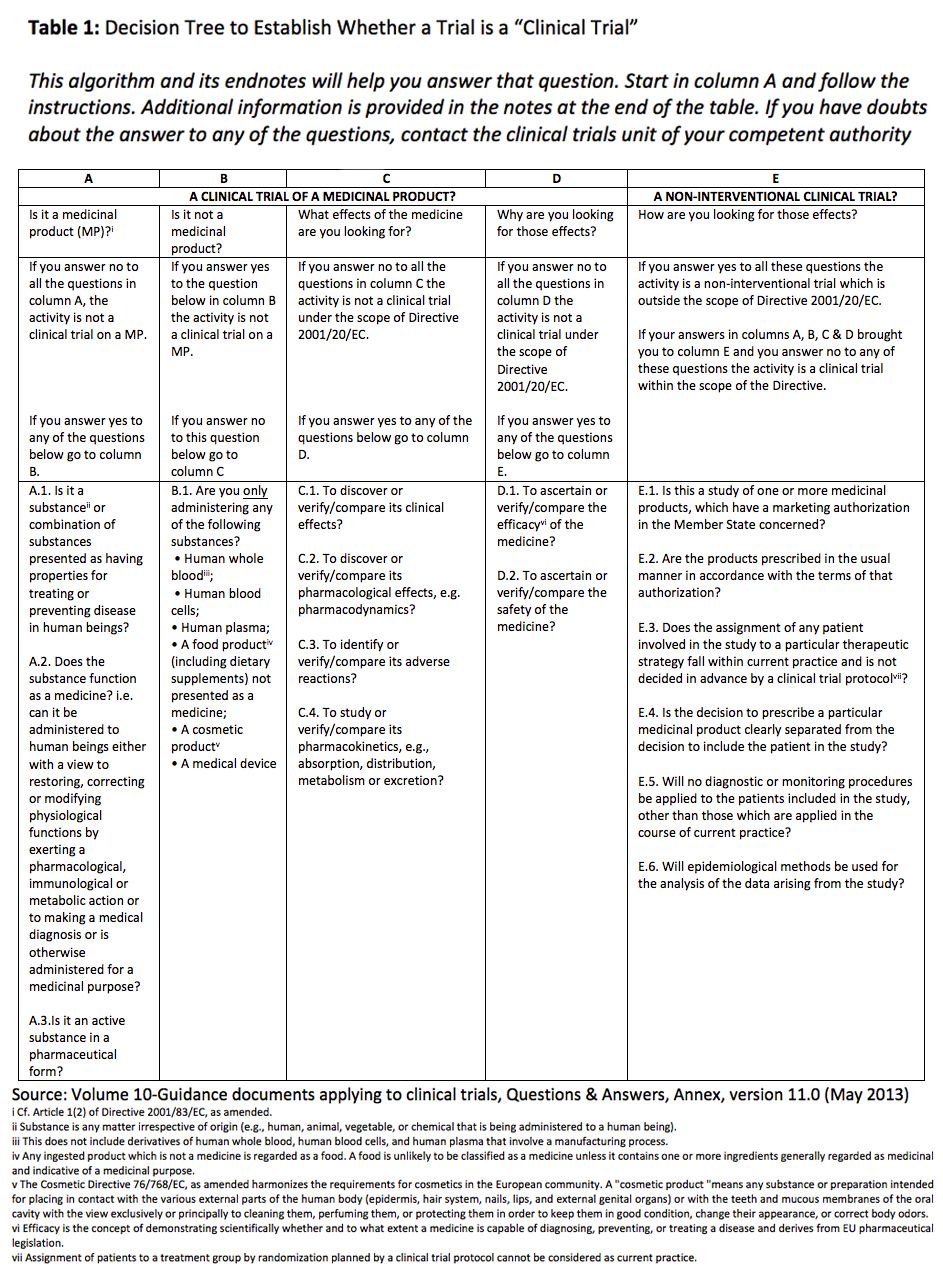
Interventional vs. Non-interventional Study Classification in the EU: Considerations on the Impact of Direct-to-Patient Contacts