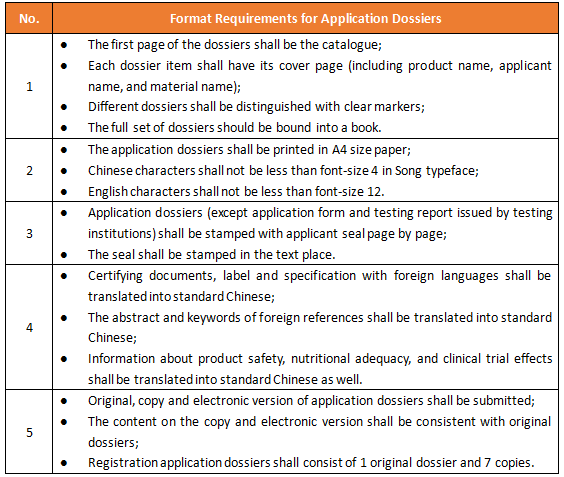
Dossier Requirements of Foods for Special Medical Purpose (FSMP) Registration in China - Regulatory News - Food & Food Contact Materials - CIRS Group

What you Need and When – The Key Documents in the Drug Lifecycle - Trilogy Writing & Consulting GmbH

Dossier System as a Practical Tool for Compiling Reimbursement Lists - Value in Health Regional Issues

What you Need and When – The Key Documents in the Drug Lifecycle - Trilogy Writing & Consulting GmbH












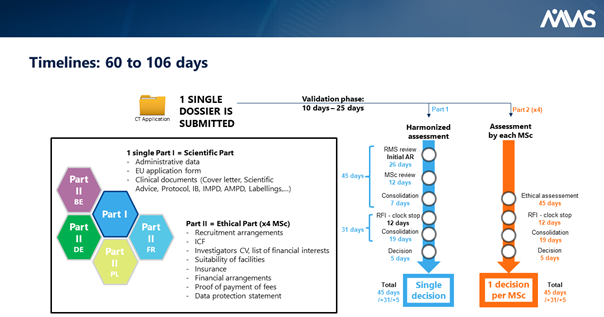




.png)
