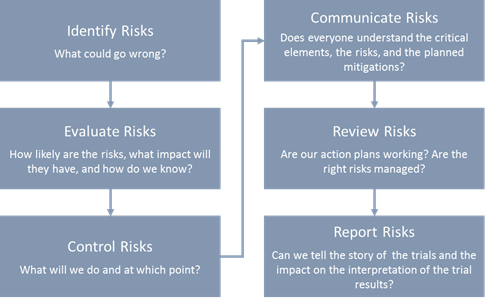
PDF) Principles and recommendations for incorporating estimands into clinical study protocol templates

White Paper: Protocol Design in Real-World Evidence: The Indispensable Link Between Strategic Need and Study Execution - Evidera
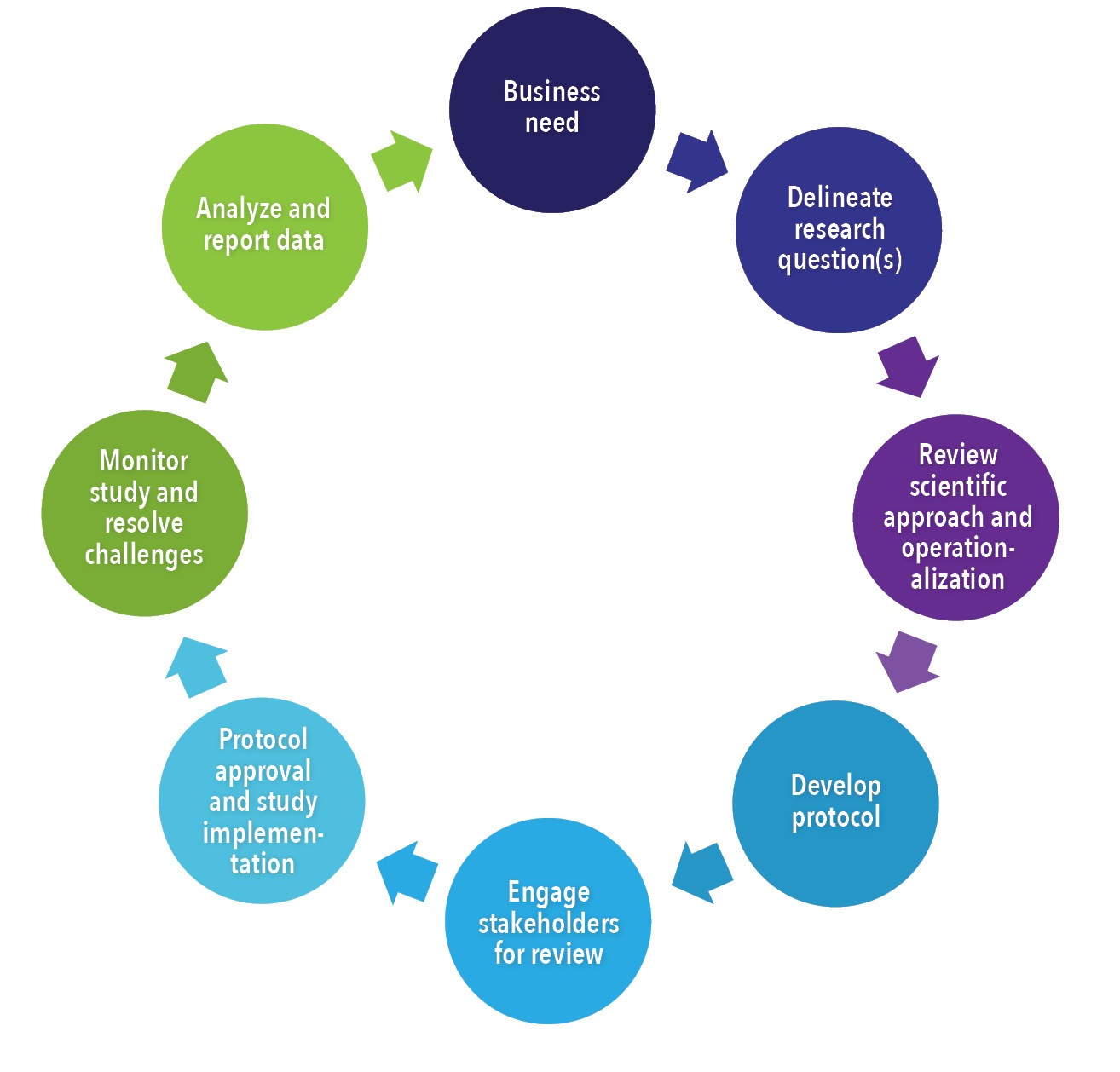
White Paper: Protocol Design in Real-World Evidence: The Indispensable Link Between Strategic Need and Study Execution - Evidera