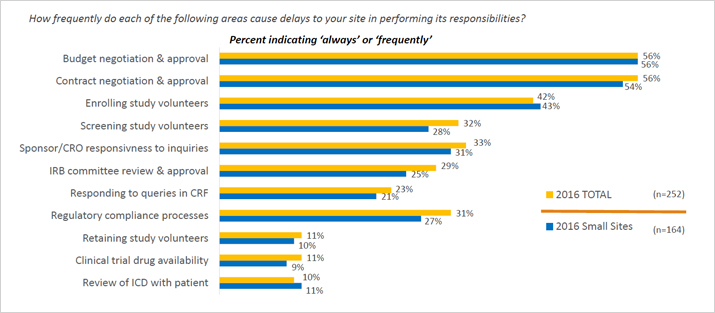
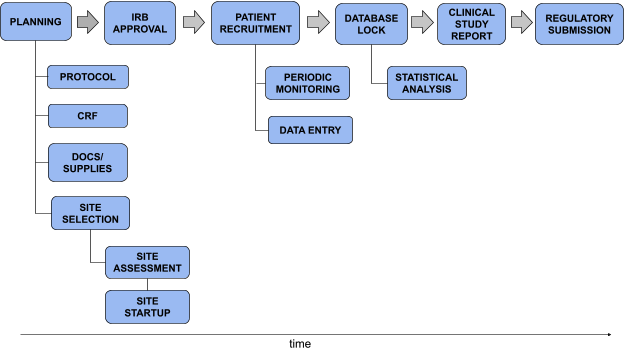
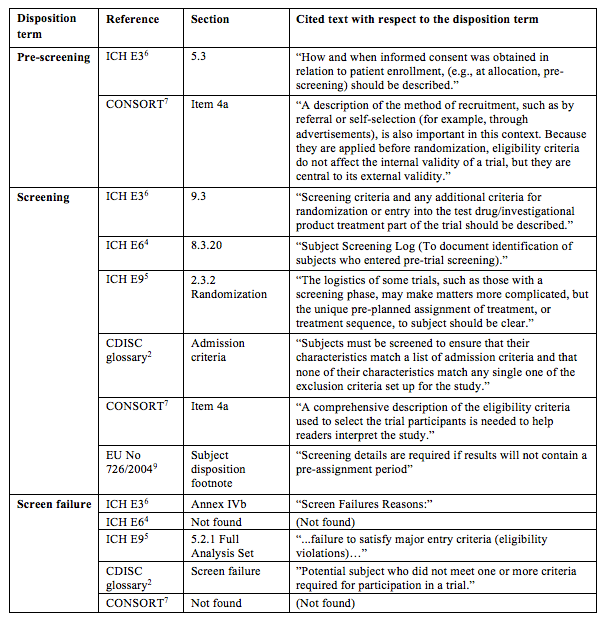
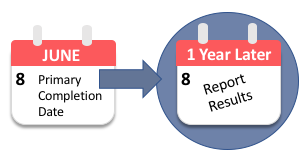
Compliance with legal requirement to report clinical trial results on ClinicalTrials.gov: a cohort study - The Lancet

Example of Clinical Trial Assessment of Infrastructure Matrix (CT AIM)... | Download Scientific Diagram

Services - Clinical Trial Report Preparation Services from Lucknow Uttar Pradesh India | ID - 3118976

Clinical Trial Report Template (6) - TEMPLATES EXAMPLE | TEMPLATES EXAMPLE | Report template, Clinical trials, Template google
Completeness of Reporting of Patient-Relevant Clinical Trial Outcomes: Comparison of Unpublished Clinical Study Reports with Publicly Available Data | PLOS Medicine




![PDF] Effective authoring of clinical study reports: A companion guide | Semantic Scholar PDF] Effective authoring of clinical study reports: A companion guide | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/2f8d38c9afbd7fce7a7635cf459a7989c82a753a/5-Table1-1.png)