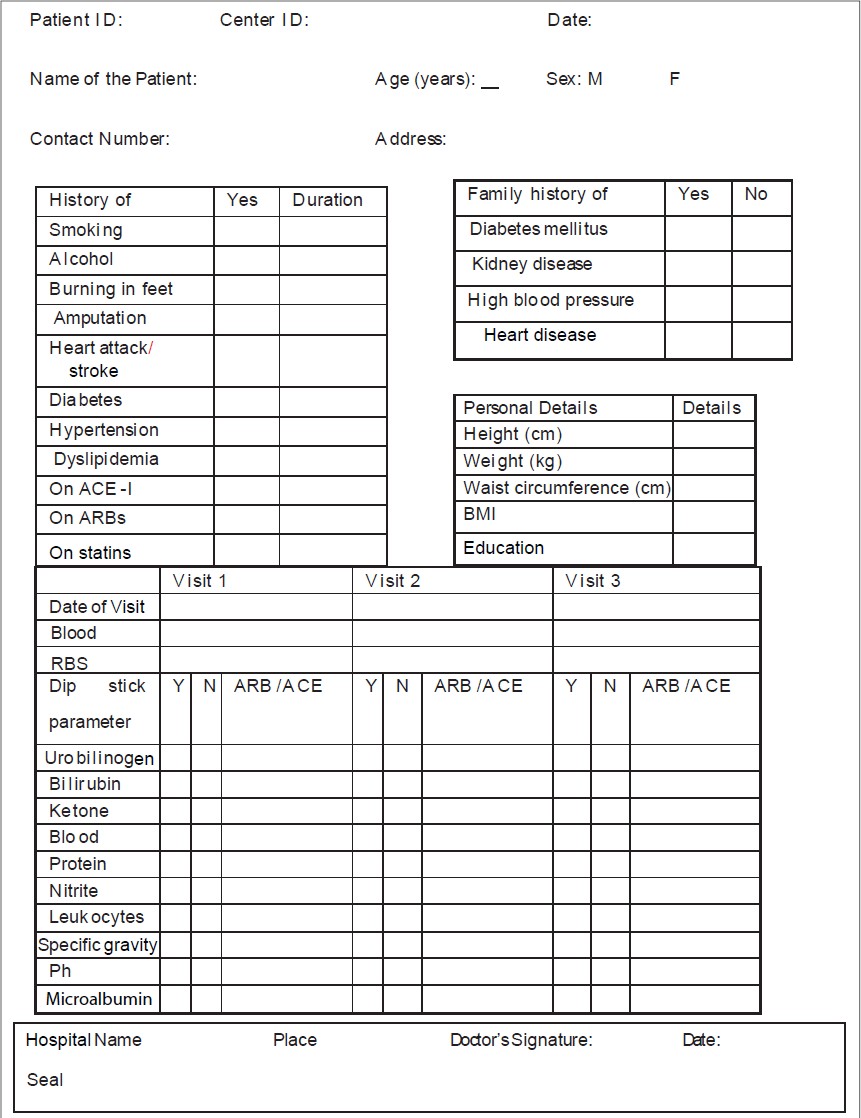
Long-term follow-up form. Simple one page CRF for collection of primary... | Download Scientific Diagram

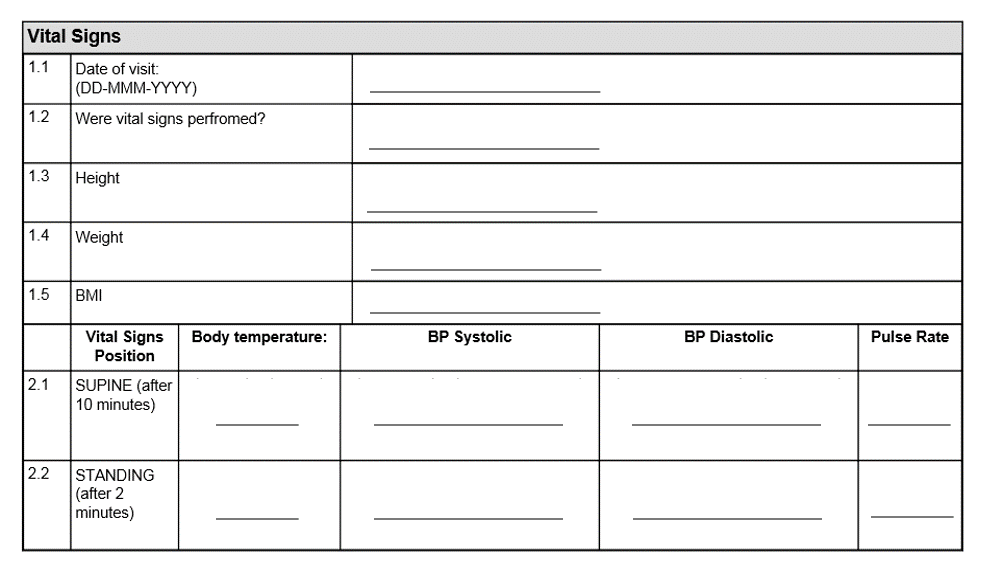
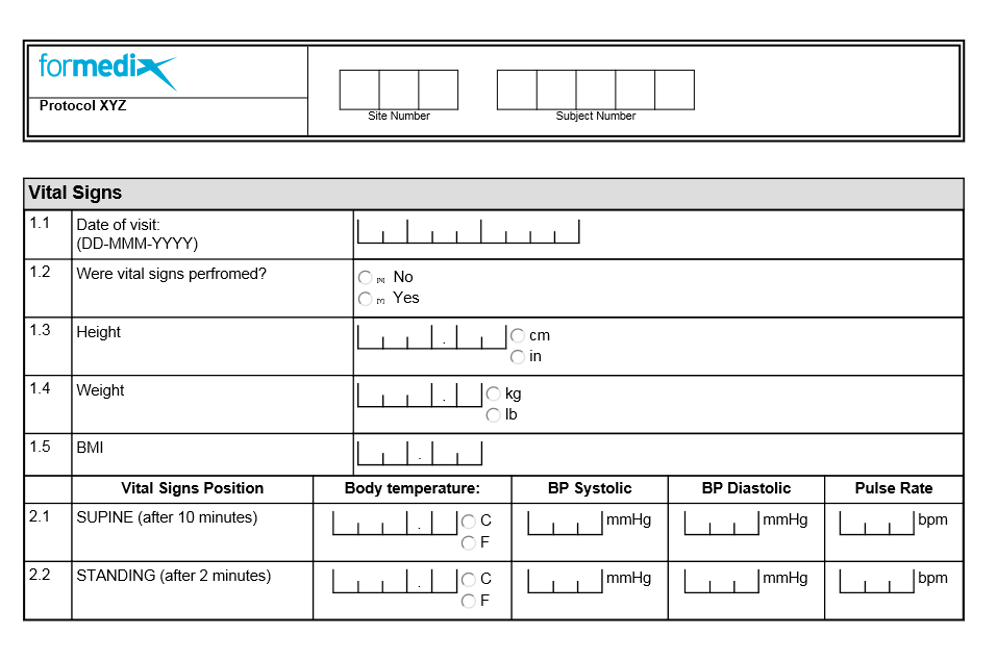
Case Report Form (CRF) for clinical examination 1. CRF to be filled by... | Download Scientific Diagram

Case Report Form (CRF) for clinical examinations 2, 3 and 4. CRF to be... | Download Scientific Diagram
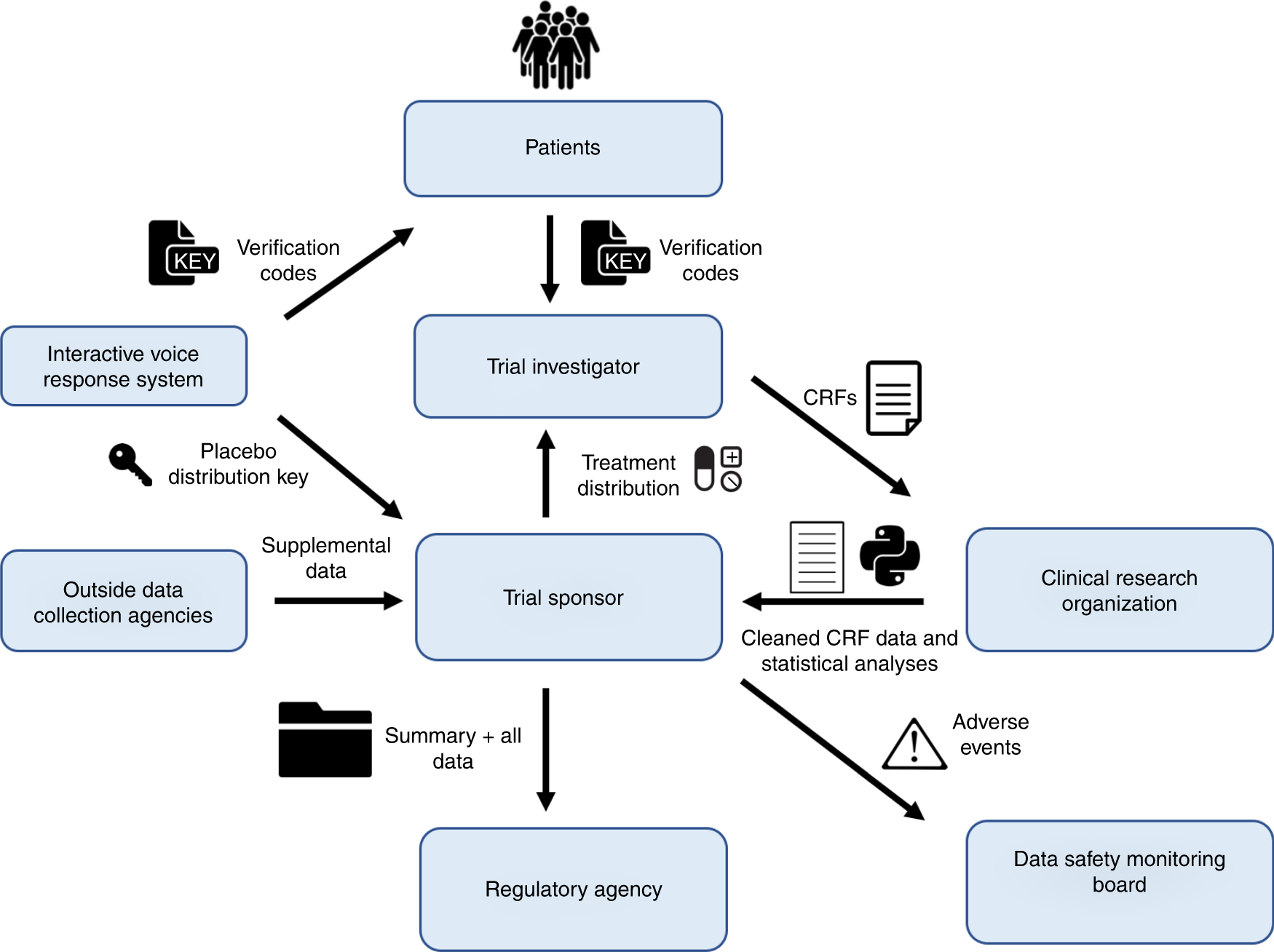
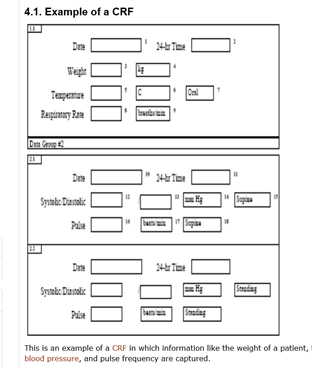
Prototype of running clinical trials in an untrustworthy environment using blockchain | Nature Communications